Project
Vision, Mission & Objectives
Advance precision psychiatry by introducing biology into clinical routine and accelerate access to treatment for people with bipolar disorder.
Objectives of the Consortium
- Objectify and aid the diagnosis of bipolar disorder thanks to EDIT-B blood test
- Make EDIT-B commercially available at the end of the EIT Health project from 2025
- Allow a swift adoption in clinical practices in all Europe
- Be a game changer for patients with mood disorder
Expected impacts:
- For patients: Improving education, work and personal life by reducing the diagnostic delay and giving fast access to the right treatment
- For society: Reducing the economic burden and optimizing use of medical and financial resources; raising awareness for bipolar disorder
- For clinicians: Introducing an aid for challenging diagnosis of bipolar disorder
- For research: Disseminating new knowledge in the fields of RNA editing and bipolar disorder
Project Overview
The EDIT-B Consortium aims to solve the bipolar disorder diagnostic challenge by using specific RNA editing based biomarkers and artificial intelligence to validate and commercialize a high performance and quick blood test to diagnose bipolar disorder.
At the beginning of the project, a kit is to be developed for the EDIT-B test. It is then clinically validated thanks to a two years clinical trial in four clinical centers. During this clinical trial, patients with depression, suffering either from bipolar disorder or from major depressive disorder, are recruited. Simultaneously, market access and commercialization strategies are built and implemented. Comprehensive communication will ensure knowledge dissemination. The objective is to ensure rapid adoption of EDIT-B after obtaining the CE mark under the new EU IVDR regulation 2017/746 (a key project objective).
The EDIT-B Consortium has a total budget of 5.2 M € and is co-funded by EIT Health (2.5 M €) and its partners. The project duration is three years.
| Work package No | Description | WP Leader |
|---|---|---|
| WP1 | Coordination and Management | Alcediag |
| WP2 | Clinical Trial | Alcediag |
| WP3 | EDIT-B kit development | Alcediag |
| WP4 | Sample Analysis | Synlab |
| WP5 | Regulatory Process | ProductLife Group |
| WP6 | Go to Market | Alcediag |
| WP7 | Dissemination, communication and education | Alcediag |
EDIT-B test
EDIT-B is a non-invasive In Vitro Medical Device (IVD), combining RNA sequencing technology related to identification of RNA editing biomarkers and artificial intelligence. It is intended for the assessment of patients suffering from depression, using whole blood as input. The test is intended to differentiate bipolar disorder from major depressive disorder (unipolar disorder) as an aid to the diagnostic. In addition to the usual diagnostic methods, such as the DSM-V, ICD 11 criteria and clinical scales such as MADRS, HDRS and BDI, the results of the test will complement the clinical and behavioural arguments usually used by the physician to establish the final diagnosis.
The following figure represents the real life process of EDIT-B test:
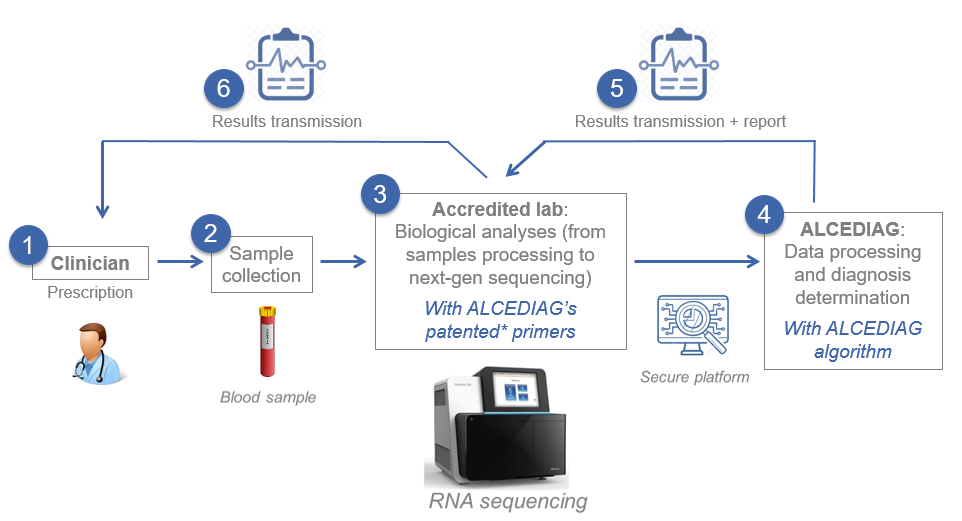
Real life process of the test EDIT-B
Clinical trial
The clinical trial to validate EDIT-B test is performed at four clinical centers that are also partners of the Consortium:
- Hospital Clínic de Barcelona (Spain); Principal investigator: Dr Eduard Vieta
- GHU Paris Psychiatrie & Neurosciences (France); Investigator : Dr Chantal Henry
- Parc Sanitari Sant Joan de Déu (Spain); Investigator: Dr Josep Maria Haro
- Psychiatric Center Copenhagen, Capital Region of Denmark (Denmark); Investigator: Dr Lars Kessing
Within two years, a total number of 436 patients with depression will be recruited, 109 in each center: 218 patients with bipolar disorder and 218 patients with major depressive disorder
Synlab Italy analyses the samples as central laboratory.